Gerald Storch, CEO of Storch Advisors and former Toys”R”Us and Target executive, reacts to a strong November retail report and explains how the industry’s winners are “value-based” players.
A Costco brand cold and flu medicine was pulled from shelves “due to potential contamination by foreign materials.”
A notice shared on the retail giant’s website states that the Kirkland Signature Severe Cold & Flu Plus Congestion recall involves item number 1729556 with specific lot code P140082, which was sold in select Midwest locations and the southeast.
“Out of an abundance of caution, LNK has issued a recall for the accidental release and shipment of a specific lot code that was rejected due to potential contamination with foreign materials,” the statement said.
The affected over-the-counter medication was purchased by customers between October 30 and November 30, 2024.
COSTCO ISSUES RECALL NOTICES FOR FROZEN WAFFLES AND FREEZE DRIED MEAT DUE TO LISTERIA CONCERNS
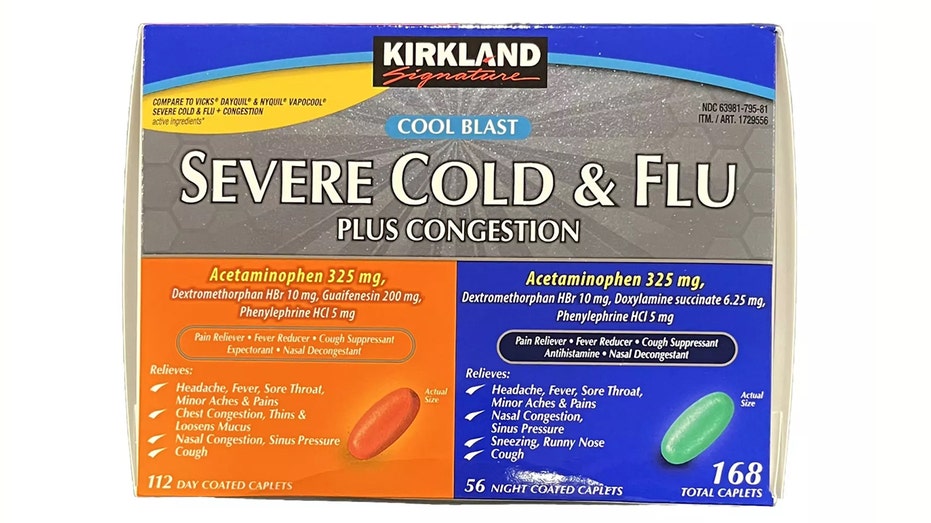
Customers who purchased Kirkland Signature Severe Cold & Flu Plus Congestion item #1729556 should check to see if they have recalled lot code e P140082. (eBay/Fox News)
Consumers are asked not to use any remaining products marked with the lot code and return them to Costco for a full refund.
Anyone with problems or concerns is asked to contact the manufacturer LNK International Inc. at 1-800-426-9391 or by email at complaints-inquiries@lnkintl.com.
Fox Business has contacted Costco for more information.
COSTCO RECALLS SEVERAL ITEMS AMID LISTERIA CONCERNS

Units affected by the January 2025 recall of the over-the-counter drug have lot code P140082 printed on the boxes. (LNK International Inc.)
| Teleprinter | Security | Last | Change | Change % |
|---|---|---|---|---|
| COST | COSTCO GROSS CORP. | 916.58 | +6.77 |
+0.74% |
Last month, 8,640 boxes of Kirkland Severe Cold & Flu Plus Congestion Day and Night packs were recalled by the Food & Drug Administration (FDA) due to the fact that the oral ingredient phenylephrine was found to be “not effective” as a nasal decongestant following an “extensive review.”
“This chemical is found to be ineffective against colds and flu in its oral form, except at a dose which has some cardiac toxicity and can lead to palpitations, arrhythmias and heart rhythm disturbances. high blood pressure,” said Dr. Marc Siegel, Fox News senior medical analyst.

Customers wait in line to pay at a Costco store on December 11, 2024 in Novato, California. (Justin Sullivan/Getty Images/Getty Images)
CLICK HERE TO LEARN MORE ABOUT FOX BUSINESS
Boxes recalled in December of the Kirkland Cold & Flu product had lot numbers P139953 or P139815 with an expiration date of August 2026.
Angelica Stabile of Fox News contributed to this report.