Find out what clicks on FoxBusiness.com.
A popular brand of eye drops is recalled throughout the country due to possible contamination, which could cause visual damage, according to the Food and Drug Administration (FDA).
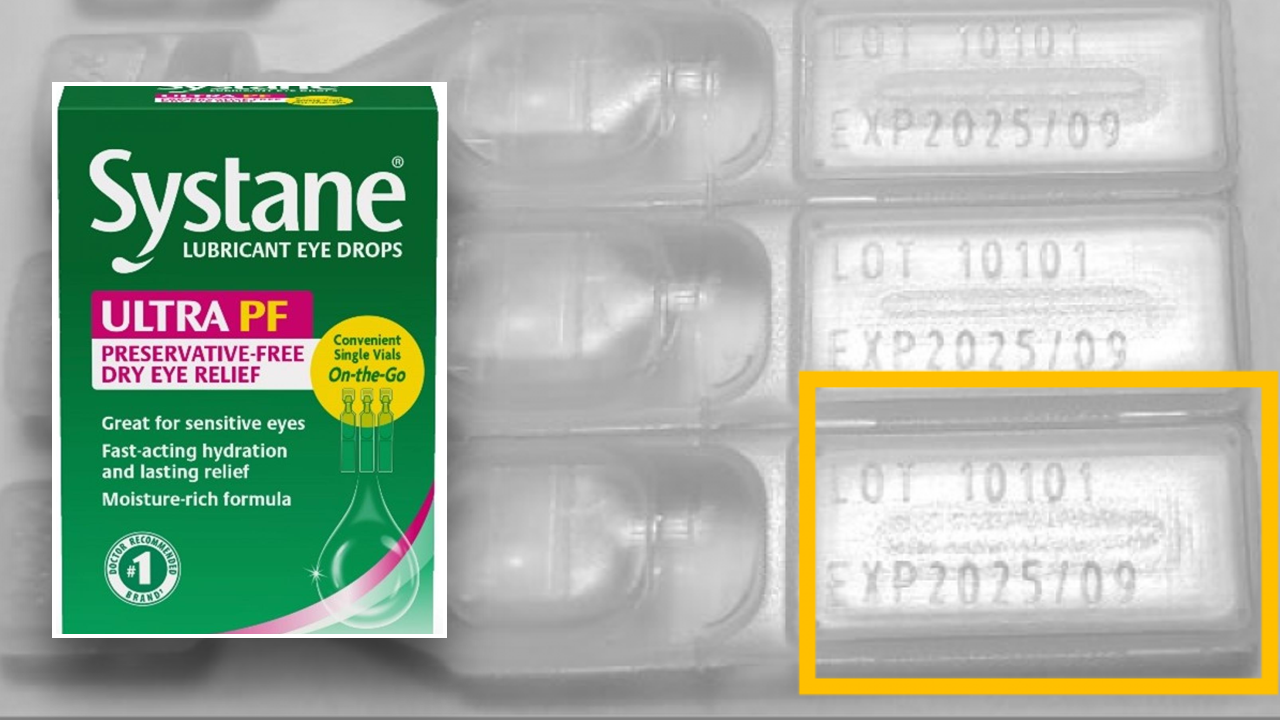
On Monday, the FDA announced that Texas-based Alcon Laboratories was voluntarily recalling a single lot of “Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go” because the products may be contaminated with fungus.
The company reported a consumer complaint that a “foreign object” was found inside a sealed, single-use bottle and determined the material was “fungal in nature.”
Fungal contamination of eye products is knowno potentially cause eye infections, the FDA said.
EYE PRODUCTS SOLD AT WALMART, CVS MAY POSE AN INFECTION RISK

The Food and Drug Administration (FDA) announced Monday that Texas-based Alcon Laboratories is voluntarily recalling a single lot of “Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go” because the products may be contaminated with fun substances. (The Food and Drug Administration (FDA) / Fox News)
If an infection occurs, the FDA said it could threaten vision and, in very rare cases, potentially be life-threatening in immunocompromised patients.
To date, the FDA said Alcon Laboratories has not received any reports of adverse reactions related to this recall.
The FDA says the affected product includes Systane Lubricant Eye Drops Ultra PF, Single Take-Away Bottles, 25 units and is limited to lot number 10101, expiration date 2025/09.
The product can be identified by the green and pink carton design, the presence of the brand names “Systane” and “ULTRA PF” on the front of the carton, and the package size “25 vials,” the FDA explained in a press release.
FDA RAISES COSTCO EGG RECALL TO HIGHEST RISK LEVEL DUE TO SALMONELLA FEARS

“Systane Ultra SPF Lubricating Eye Drops, Single On-The-Go Bottles” have been recalled because the products may be contaminated with fungus, according to the FDA. (The Food and Drug Administration (FDA) / Fox News)
Most of the potentially affected eye drops were also distributed nationwide to retail outlets and over the Internet.
Consumers who possess the recalled eye drops are urged to stop using them immediately and return them to the place of purchase for a replacement or refund, the FDA said.
Distributors or retailers who have the recalled eye drops are also asked to dispose of any remaining stock of the contaminated product.
Alcon Laboratories also notifies its distributors and customers by letter, email and/or telephone call and arranges for replacement of all recalled products.
BELOVED SNACK BRAND RECALLS ‘LIMITED’ NUMBER OF POPULAR CHIPS BAGS DUE TO UNDECLARED ALLERGEN: FDA

Alcon Laboratories issued the recall after a consumer complained of a “foreign object” found in a sealed bottle of eye drops. (The Food and Drug Administration (FDA) / Fox News)
This latest recall comes as several eye products have been pulled from shelves over the past year due to the potential risk of infection.
In February, eye ointments sold at CVS and Walmart stores nationwide were recalled after the FDA discovered a “lack of sterility assurance” at the manufacturing plant.

Alcon Laboratories in Texas is voluntarily recalling Systane Ultra PF Lubricating Eye Drops following a consumer complaint regarding foreign material that may be observed inside a sealed bottle. (The Food and Drug Administration (FDA) / Fox News Latino)
The four affected products, which are intended to be sterile, are sold under the brands Equate, CVS Health and AACE Pharmaceuticals, and have expiration dates ranging from February 2024 to September 2025. The products were distributed nationwide to the wholesalers, to retailers and through the product distributor, Walmart, CVS and AACE Pharmaceuticals Inc.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
In November 2023, Kilitch Healthcare India Limited voluntarily recalled 27 eye drops, all of which were on the FDA’s rolling list of products that may be contaminated with bacteria, posing a risk of eye infection and vision loss.
None of the recalls mentioned above are linked to the outbreak of antibiotic-resistant bacteria. pseudomonas aeruginosa related to eye products by Global Pharma Healthcare.
Daniella Genovese of Fox News Digital contributed to this report.